Abstract
BACKGROUND: HyperCVAD is used commonly in adult ALL but can be difficult for older or infirm patients (pts) to tolerate (JCO 2000, p. 547; Cancer 2008, p. 2097). At our center, only 50% who receive it reach a minimal residual disease negative (MRD-) state within 90 days (Am J Hematol 2018, p. 546). Lower-intensity options with ABL tyrosine kinase inhibitors (TKIs) yield high response rates in Philadelphia chromosome (Ph)+ disease, but long-term efficacy may be limited (Blood 2016, p. 774; ASH 2017, #99). DA-EPOCH is effective and relatively well-tolerated in high-grade lymphomas (NEJM 2013, p. 1915; Blood 2014, p. 2354). This prompted us to study DA-EPOCH as initial treatment of adult ALL.
METHODS: Pts ≥ 18 years old with newly-diagnosed ALL were eligible if they were not a candidate for pediatric-inspired therapy (e.g., Ph+, age ≥ 40) and had adequate liver and kidney function. All pts signed IRB-approved consent forms. This single-center trial is registered on clinicaltrials.gov (NCT03023046) and supported by NCI 5P30 CA015704-41. DA-EPOCH was given as initially described (Blood 2002, p. 2685). Doses of myelosuppressive agents were adjusted based on the hematologic nadir after the previous cycle, but pts could start the next cycle before day 21 once ANC ≥ 1K/μL and platelets (plt) ≥ 50K/μL, and (after 1st 5 pts treated) dose-reduction was deferred if cytopenias reflected ALL. If Ph+, imatinib (IM) 600 mg or dasatinib (DAS) 100 mg daily on Days 1-14 of each cycle was added. If CD20+, rituximab (R) 375 mg/m2 was given once per cycle. All pts received G-CSF and intrathecal prophylaxis.
Response was determined by morphology (morph), multiparameter flow cytometry (MFC) and (for Ph+) BCR-ABL RT-PCR on bone marrow aspirate (BMA). When MRD- by MFC (MFC-), high-throughput sequencing (HTS)-based MRD testing was performed as described previously (Clin Cancer Res 2014, p. 4540). BMA was performed after cycle 1, then repeated as needed to confirm MFC- or persistence. Up to 8 cycles could be given followed by maintenance (POMP ± TKI) or hematopoietic cell transplant (HCT) at physician's discretion, though morph CR after ≤ 2 cycles was needed to continue.
The primary endpoint was rate of MFC- after ≤ 4 cycles (i.e., < 90 days), defined by EuroFlow criteria (Leukemia 2010, p. 521). Using a historical rate with hyperCVAD of 50% (cited above) and defining success if observed rate is 70%, a Simon 2-stage design with α = 0.09 and 80% power led to the following: total sample size of 28 pts, stop enrollment if ≤ 7 of 15 were MFC- after ≤ 4 cycles, and judge the regimen of interest if ≥ 18 of 28 were MFC- after ≤ 4 cycles. Follow-up provided to 7/10/18.
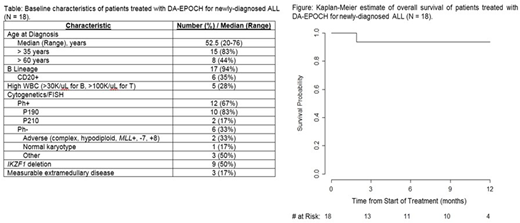
RESULTS: 18 pts have received ≥ 1 cycle (Table). 15 (83%) achieved morph CR after ≤ 2 cycles. 8 of 14 (57%; 95% CI 0.29-0.82) evaluable for the primary endpoint were MFC- after ≤ 4 cycles; 3 pts remain too early to evaluate; 1 pt was removed after 2 cycles to continue DA-EPOCH closer to home. 2 Ph+ pts received IM, and 10 received DAS. 7 of 9 (78%) evaluable Ph+ pts were MFC- after ≤ 4 cycles, 3 after 1 cycle. 6 Ph+ pts (5 p190 and 1 p210; 67%) were MRD- by PCR after ≤ 4 cycles. 1 of 5 evaluable Ph- pts (20%) reached MFC- after 1 cycle. MRD by HTS was sought in 5 MFC- patients: clonal sequence was identifiable pre-treatment in 4, and 3 (75%) achieved MRD- by HTS, including 2 after only 1 cycle (both Ph+).
16 (89%) pts developed Grade (Gr) 3+ non-hematologic toxicity at some point throughout treatment, with febrile neutropenia (7 Gr 3), hypofibrinogenemia (4 Gr 3), and GI bleed (2 Gr 3, 1 Gr 5) the most common. 1 pt (6%) died in morph CR after Cycle 3; no other pts stopped due to toxicity. Median cycle length was 21 days (range: 16-27). Once beyond Cycle 2, only 7 of 28 total cycles (25%) yielded ANC < 500/μL for ≥ 1 week or any plt < 25K/μL (i.e., dose-reduction per the DA-EPOCH paradigm).
5 pts (28%) have undergone HCT, 3 of whom were in CR1 after DA-EPOCH (all Ph+). 5 in CR after DA-EPOCH have relapsed (33%), 1 of whom was post-HCT. No deaths from ALL have occurred. Median and 6-mo EFS are 8 mo and 61% (respectively). Median OS is not reached, and 6-mo OS is 94% (Figure).
CONCLUSIONS: DA-EPOCH yields deep remissions (including by HTS) in newly-diagnosed ALL, particularly with TKI for Ph+ disease. Toxicity is manageable and rarely leads to discontinuation or delay. Because of the relatively high rate of early MFC-, the interim stopping rule for efficacy was not met, and enrollment continues. Updated results (including genomic profiling by RNAseq) will be presented.
Cassaday:Kite Pharma: Research Funding; Pfizer: Consultancy, Research Funding; Merck: Research Funding; Amgen: Consultancy, Research Funding; Incyte: Research Funding; Jazz Pharmaceuticals: Consultancy; Adaptive Biotechnologies: Consultancy; Seattle Genetics: Other: Spouse Employment, Research Funding. Shustov:Seattle Genetics: Research Funding. Becker:GlycoMimetics: Research Funding. Radich:TwinStrand Biosciences: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal